The combustion process between propane and oxygen is used as an example to demonstrate this method. In secondary chemistry education two predominant methods are typically employed to convey a systematic method of chemical reaction balancing.

What Is The Chemical Equation For Cellular Respiration Video Lesson Transcript Study Com
When it comes to balancing chemical equations the first step is to obtain the full imbalanced equation.
. Which lists the numbers of each atom the other half of the equation would contain. Let us consider the formation of water from the combination of oxygen and hydrogen. This method is used for simpler problems.
Balancing Chemical Equations recognize that the number of atoms of each element is conserved in a chemical reaction. A coefficient number in front of a chemical is multiplied by all the atoms in that chemical. Isopropanol burns in oxygen to yield carbon dioxide water and heat.
Akia is balancing the equation Na H2O NaOH H2. The mass of the reactants and products is equal and is not dependent on the physical state of the substances. The Algebraic Balancing Method This method of balancing chemical equations involves assigning algebraic variables as stoichiometric coefficients to each species in the unbalanced chemical equation.
In the Balancing by Numerical method a reaction is balanced and the coefficients can be seen. Coefficients in a chemical equation express ________________ between molecules or compounds. 3 Mg 2 P 14 O 12 H.
Balancing by inspection is one of the basic and simple methods to balance the chemical equation. 1 2 2 1 4. The coefficient is a number that goes in front of a chemical formula.
The following table shows the number of. Write the balanced chemical equation for the decomposition of sodium azide. Terms in this set 10 Which best describes the law of conservation of mass.
Hence the Balancing by Numerical method is mostly used as it describes if the equation is balanced or not. AnswerhiiExplanationquestion is incompletebut to balance any equation u should satisfy that equation with law of mass conservationI hope this will help you. Therefore the balanced chemical equation is C3H8 5O2 3CO2 4H2O.
What does the chemical formula C3H6O2 tell about a molecule of the compound it represents. I-Br2 --- IO3-Br- Step 1. First note down the elements on each side of the equation and their atoms.
The following sequential steps be taken to obtain a balanced chemical equation. 4 4 yes. 3H2 g N2 gNH3 g If three molecules of hydrogen react with one molecule of nitrogen how many molecules of ammonia would be formed.
2 2 4. SnO 2 H 2 Sn 2 H 2 O. A subscript lower number is only.
The steps for balancing a chemical equation are. I will leave the first one for you to do. The chemical equation for the formation of ammonia is unbalanced.
Which best describes Mins method for balancing the chemical equation. Br2 Br- I- IO3- Step 2. When balancing equations remember chemical reactions must satisfy conservation of mass.
To get four hydrogen atoms on the right add a coefficient of 2 for the hydrogen gas. This balancing method of chemical equations includes assigning algebraic variables as stoichiometric coefficients to every species in the unbalanced chemical equation. This puts the hydrogen atoms out of balance.
The same number of atoms of each element must exist on the reactant side. Rules of the Game 1. For example H2 O2 H2O Caution.
Which best describes Mins method for balancing the chemical equation. It is incorrect because the. Obtain the unbalanced equation from the chemical formula of the reactants and the products.
The chemical formula of propane is C3H8. C 3 H 8 O 2 CO 2 H 2 O. Ii The same total of charges should appear on the.
156K people helped. This is important because a chemical equation must obey the law of conservation of mass and the law of constant proportions ie. The second one is.
General objective of this lecture is to explain on Balancing Chemical Equations. I First write the skeleton equation. A Balanced Equation CH4 2O2 CO2 2H2O Reactant Side Product Side 1 carbon atom 4 hydrogen atoms 4 oxygen atoms 1 carbon atom 4 hydrogen atoms 4 oxygen atoms.
Passwod4548 passwod4548 09042020 Science Secondary School Min is asked to balance the equation below Which best describes Mins method for balancing the chemical equation question 1. Break the reaction into the two half reactions. Matter cannot be created or destroyed.
3Mg OH2 2H3PO4. You can only change coefficients. One half of a balanced chemical equation is shown.
Balancing Chemical Equations Quiz. Using the half- reaction method balance the redox reaction shown below. --- Balancing Chemical Equations in Five Easy Steps --- Balancing chemical equations is a core skill in chemistry.
Method to teach the skill set needed to perform the task of chemical reaction balancing in the secondary education setting. Pick one element which isnt equal on both sides of the equation and add a coefficient to the formula with the equation to make it equal. This burns with oxygen O2 to form carbon dioxide CO2 and water H2O The unbalanced chemical equation can be written as.
Subscripts cannot be added removed or changed. Check your work to make certain you have the same number and type of atoms on the reactants side as on the products side. The Algebraic Balancing Equations Method.
In this video youll learn the basics for. By inspection a or b with a linear algebraic method 67. Which word equation best represents this chemical reaction.
The unbalanced chemical equation can be written as C 3 H 8 O 2 CO 2 H 2 O. Do not change formula of any constituent while balancing the equation. Now there are two hydrogen atoms on the left and four hydrogen atoms on the right.
This system describe the difference between coefficients and subscripts in a chemical equation. And these variables are used in mathematical equations and solved to obtain the values of each stoichiometric coefficient. Balancing chemical equations involves the addition of stoichiometric coefficients to the reactants and products.
It is incorrect because the identity of the original product has changed. In terms of Algebraic Solving Method. It is made up of 11 total atoms of 3 different elements.

Using Evidence To Determine The Correct Chemical Equation A Stoichiometry Investigation Chemical Education Xchange

Using Evidence To Determine The Correct Chemical Equation A Stoichiometry Investigation Chemical Education Xchange

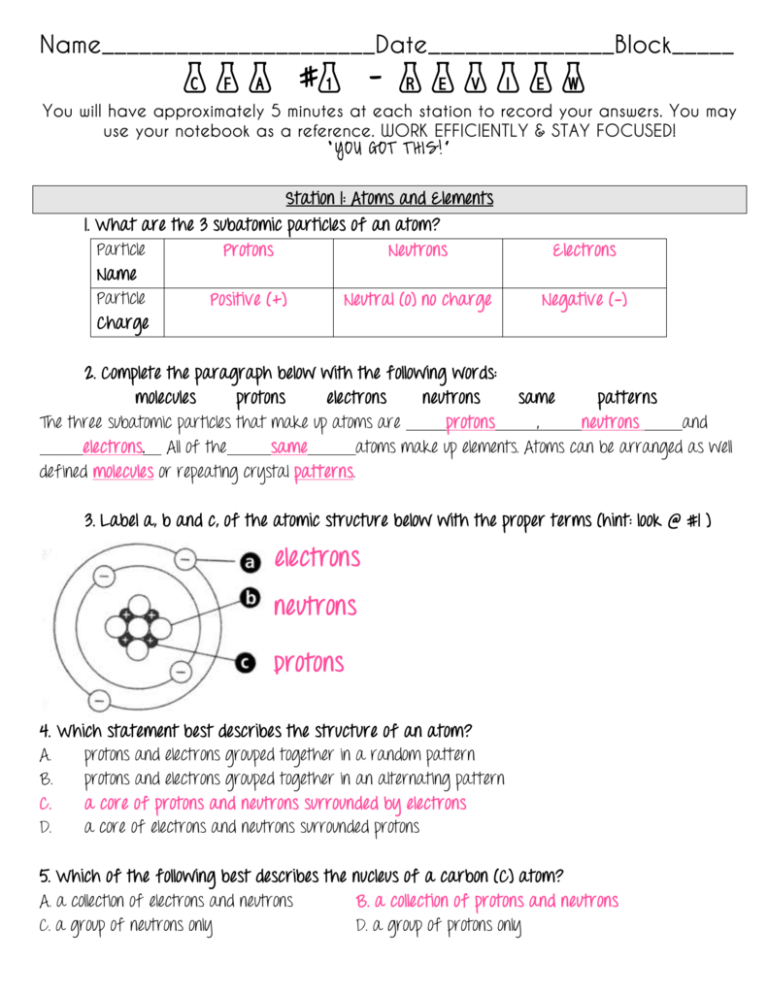
Cfa 1 Review Key Science With Mrs Barton

Using Evidence To Determine The Correct Chemical Equation A Stoichiometry Investigation Chemical Education Xchange
0 Comments